Inter-university Accelerator Centre, New Delhi, India
Heavy Ion Radiation Biology is an interdisciplinary applied science involving Atomic and Nuclear Physics, Molecular Biology and Biochemistry. The current research in this field investigates the effects of accelerated charged particles on the biological systems at the molecular level. In that sense, the research field that is intended to be pursued at IUAC may be termed Molecular Radiation Biology.
The Radiation Biology research facility in terms of a dedicated beamline and a molecular biology laboratory is very much suitable for carrying out fundamental research in this field keeping up pace with the other laboratories in the world. Various experiments can be designed to study the molecular mechanisms leading to the radiation-induced effects.
Various experiments may be planned by choosing suitable projectiles with appropriate energies to irradiate the samples of interest. The dedicated beamline for Heavy Ion Radiation Biology is designed for experiments using the low flux of accelerated particles. The irradiation is done using a computer-controlled system called ASPIRE. The support laboratory includes a cell culture facility and other equipment for sample preparation and post-irradiation treatments and analysis.
The obtained data may be used for :
- Modeling radiation effects
- Heavy Ion Cancer therapy
- Radiation protection in manned space flights
- Exploring different pathways to radiation induced effects
Research Programmes
- Effect of 45 MeV 7Li and 68 MeV 16O charged particles on the microsomal membrane fluidity. M. Srivastava, D. Choudhary, A. Sarma and R.K. Kale, Current Science, 74, No.1(1998) 58
- Effect of high LET radiation on biological membranes. D. Choudhary, M. Srivastava, A. Sarma and R.K. Kale, Radiat Environ Biophys, 37, No.3(1998), 177-185
- Response to glyoxalase system to low doses of mixed radiation. D. Choudhary, D.Chandra, S.P. Lochab, A. Sarma and R.K. Kale. Physica Medica, Vol XV, N.1, January - March 1999, page 27-33
- Effect of 7Li (45 MeV) ions on spinach leaves studied by thermoluminescence technique. Jyoti Gaikwad, S. Thomas, S. Kamble, P.B. Vidyasagar and A. Sarma, Nucl. Instr. And Meth. In Phys. Res. B 156 (1999) 231-235
- Effect of ionizing radiation: induced damage and protection in normal and denatured supercoiled DNA and minichromatins formed on them. S. De, S. Ray Chaudhuri, A. Sarma and A.R. Thakur, Indian J. Phys. 73B (5), 1999, 795 – 804
- Study of Carbonaceous Clusters in Irradiated Polycarbonate with UV-vis Spectroscopy, S. Gupta, D. Choudhary, A. Sarma, Journal of Polymer Science; Part B: Polymer Physics, Vol. 38, 2000, 1589 – 1594
- Effect of low doses of n-g mixed radiation on bioreductive enzymes in different tissues of mice. : D.Chandra, D.Choudhary, S.P.Lochab, A.Sarma, R.K.Kale, Physica Medica, Vol XVI, No.2, April-June 2000, page 83-87.
- Alpha-tocopherol protection against oxidative membrane damage induced by high LET radiation, Choudhary D., Sarma A, Kale RK, (2000), Research Communications in Biochemistry, Cell and molecular Biology, 4(3-4), 245-258.
- Irradiation of Thymine by 16O heavy ion in aerated condition. Pratyush Purkayastha, A.N. Talukdar and A. Sarma, Indian Journal of Pure and Applied Physics, Vol.39, November 2001, p 691-693
- Response of the FBX system to carbon beam: its potential as a dosimeter in heavy particle radiotherapy, MK Semwal, Milan Banerjee, Asiti Sarma and PB Vidyasagar, Phys. Med. Biol. 47 (2002), N179-N183
- Biological effects induced by swift heavy ions of Lithium on aqueous solution of plasmid pMTa4, J.O. Humtsoe, F.H.A. Schneeweiss, A. Srivastava, A. Sarma, R.N. Sharan, Radiation Effects and Defects in Solids, August 2003, Vol 158, p 603-607
- Proton and gamma ray induced gain degradation in bipolar transistor, S.R. Kulkarni, Asiti Sarma, G.R. Joshi, M. Ravindra and R. Damle, Radiation Effects and Defects in Solids, August 2003, Vol 158, p 647-654
- Optical absorption and photoluminescence studies on thin films and bulk crystals of LiF under swift heavy ion irradiation, F. Singh, A. Sarma, R.M. Montereali, F. Bonfigli, G. Baldacchini, D.K. Avasthi, Radiation Measurements, 2003, Vol 36, p 675-679
- Response of FBX Dosimeter to high LET 7Li and 12C ions, N. N. Bhat, D. Choudhary, A. Sarma, B.L. Gupta and K. Siddappa, : Radiation Physics and Chemistry, 68 (2003), 909-916
- Effect of Heavy Ion Irradiation on DNA DSB Repair in Methanosarcina Barkeri. Ray chaudhury S, Karmakar P, Choudhary D, Sarma A, Thakur A R, Anaerobe 2003 Feb;9(1):15-21
- Assessment of 1H heavy ion irradiation-induced effects in the development of rice (Oryza sativa L.) seedlings, M. Kalimullah, J.U. Gaikwad, S. Thomas, A. Sarma, P.B. Vidyasagar, Plant Science 165 (2003), 447-454
- Expression of NF-kappaB and ERK following heavy ion irradiation. Mitra AK, Sarma A, Krishna M, Verma NC, J Environ Pathol Toxicol Oncol 2004;23(1):53-9.
- Alteration in the expression of signaling parameters following carbon ion irradiation. Mitra AK, Bhat N, Sarma A, Krishna M, Mol Cell Biochem. 2005 Aug;276(1-2):169-73.
- Response to high LET radiation 12C (LET, 295 keV/microm) in M5 cells, a radioresistant cell strain derived from Chinese hamster V79 cells. Pathak R, Sarma A, Sengupta B, Dey SK, Khuda-Baksh AR, Int J Radiat Biol. 2007 Jan;83(1):53-63.
- Genotoxic effects in M5 cells and Chinese hamster V79 cells after exposure to 7Li-beam (LET=60 keV/microm) and correlation of their survival dynamics to nuclear damages and cell death. Pathak R, Sarma A, Dey SK, Khuda-Baksh AR Mutat Res. Genetic Toxicology and Environmental Mutagenesis 2007 Mar 30;628(1):56-66.
- Effect of 7Li radiation on the endogenous hormonal level on developing cotton fiber. Kunjal Bhatt, Asiti Sarma & Vrinda Thaker, Indian Journal of Experimental Biology Vol. 46, September 2008, pp. 677-680
- The influence of reduced glutathione on chromosome damage induced by X-rays or heavy ion beams of different LETs and on the interaction of DNA lesions induced by radiations and bleomycin. Geetanjali Pujari, A. Sarma, A. Chatterjee, Mutation Research, Genetic Toxicology and Environmental Mutagenesis 696 (2010) 154-159
- Activation of DNA damage response signaling in lung adenocarcinoma A549 cells following oxygen beam irradiation. Ghosh S, Narang H, Sarma A, Kaur H, Krishna M, Mutation Research, 2011 Aug 16;723(2):190-8. Epub 2011 May 14.
- DNA damage response signaling in lung adenocarcinoma A549 cells following gamma and carbon beam irradiation. Ghosh S, Narang H, Sarma A, Krishna M. Mutat Res. 2011 Nov 1; 716(1-2):10-9. Epub 2011 Aug 3.
- In vitro studies on radiosensitization effect of glucose capped gold nanoparticles in photon and ion irradiation of HeLa cells, Harminder Kaur, Geetanjali Pujari, Manoj K. Semwal, Asitikantha Sarma, Devesh Kumar Avasthi, Nuclear Instruments and Methods in Physics Research B 301 (2013) 7–11
- Study of in vitro toxicity of glucose capped gold nanoparticles in malignant and normal cell lines Harminder Kaur, Geetanjali Pujari, Asitikantha Sarma, Yogendra Kumar Mishra, Mi Kyung Jin, Bikesh K. Nirala, Nivedita K Gohil, Rainer Adelung, Devesh Kumar Avasthi, Advanced Materials Letters, Volume 4, Issue 12, Page 888-894
- Radiosensitivity and Induction of Apoptosis by High LET Carbon Ion Beam and Low LET Gamma Radiation: A Comparative Study. Ghorai A, Bhattacharyya NP, Sarma A, Ghosh U. Scientifica (Cairo). 2014;2014:438030. doi: 10.1155/2014/438030. Epub 2014 Jun 15
- Carbon ion beam triggers both caspase-dependent and caspase-independent pathways of apoptosis in HeLa and status of PARP-1 controls intensity of apoptosis. Ghorai A, Sarma A, Bhattacharyya NP, Ghosh U. Apoptosis. 2015 Apr;20(4):562-80. doi: 10.1007/s10495-015-1107-3.
- ASPIRE An automated sample positioning and irradiation system for radiation biology experiments at Inter-University Accelerator Centre, New Delhi, Ashok Kothari, P. Barua, M. Archunan, Kusum Rani, E.T. Subramanian, Geetanjali Pujari, Harminder Kaur, V.V.V. Satyanarayanan, Asitikantha Sarma, D.K. Avasthi, Radiation Measurements, 76, May 2015, Pages 17–22
- PARP-1 depletion in combination with carbon ion exposure significantly reduces MMPs activity and overall increases TIMPs expression in cultured HeLa cells, Ghorai A, Sarma A, Chowdhury P, Ghosh U., Radiation Oncology, 2016 Sep, 22;11(1), 126
- Targeting DNA repair with PNKP inhibition sensitizes radioresistant prostate cancer cells to high LET radiation, Srivastava P, Sarma A, Chaturvedi CM, PLoS One, 2018 Jan, 13(1): e0190516
- 12C-Beam Induces More Chromosomal Damage In Chemo-Radio-Resistant Cells than 16O-Beam, Utpal Ghosh, Regina Lichti Binz, Ratan Sadhukhan, Asitikantha Sarma, Subrata Kumar Dey, Martin Hauer-Jensen, Rupak Pathak, Research and Reviews: Journal of Pharmacy and Pharmaceutical Sciences, May 2018, Vol 7, Issue 2, p 9
- A12B4C3, an inhibitor of polynucleotide kinase/phosphatase enhances radio-sensitivity in PC-3cells exposed to carbon ion beam, Pallavi Srivastava, Asitikantha Sarma, Chandra Moini Chaturvedi, Journal of Innovations in Pharmaceutical and Biological Sciences (JIPBS), 2018, Vol 5(2), p27-31
- Reduction of metastatic potential by inhibiting EGFR/Akt/p38/ERK signaling pathway and epithelial-mesenchymal transition after carbon ion exposure is potentiated by PARP-1 inhibition in non-small-cell lung cancer, Chowdhury P, Dey P, Ghosh S, Sarma A, Ghosh U, BMC Cancer, 2019 Aug, 22;19(1):829
Signaling pathways of activation and secretion of Matrix Metalloproteinases from human lung carcinoma cells after irradiation with carbon ion beam. [Kalyani University]
Lung malignancy is the foremost cause of cancer-related deaths globally and most of the lung tumors are inoperable and the only choice is radio or chemotherapy. The majority of death occurs due to the highly metastatic nature of lung cancer. Matrix metalloproteinases (MMPs) are a key element in metastasis and their expression is extremely high in lung tumors compared with non-malignant lung tissue. Low LET photon radiation, in contrast to carbon ion radiation, can enhance the metastatic nature of cancer cells by increasing the expression and activities of MMPs leading to great difficulties in radiotherapy using gamma or X-rays. The signaling pathways EGFR/Akt/p38/ERK associated with activation of MMP-2 and MMP-9 in highly metastatic lung carcinoma A549 cells is studied after irradiation with carbon ion beam in present and absence of different PARP-1 inhibitors.
Radiosensitisation of human cancer cells using G-quadruplex ligands. [Kalyani University]
DNA G-quadruplexes are tertiary DNA structures that are formed by Hoogsteen base pairing between four Guanine residues. They are prevalent in G rich regions of human DNA like the telomere and promoter region of some oncogenes. G- quadruplex ligands can produce DNA damages and can inhibit the synthesis of some oncoproteins. Now a day’s DNA G- quadruplexes become a potential target for cancer drugs and many G-quadruplex ligands are now under clinical trial. On the other hand, Carbon ion beam has several advantages over conventional ionizing radiation in cancer treatment. Here the possibility of enhancing the efficacy of C beam therapy is being studied through radiosensitizing human lung cancer cell A549 with a well known G-quadruplex ligand Pyridostatin (PDS)
High linear Energy Transfer Radiation-Induced autophagy in vitro model system: Dynamics of autolysosome formation [IUAC]
Autophagy is the waste disposal system of the cell, a cellular self-eating process that uses lysosomes. The basic idea behind autophagy is that the body begins to eat itself (auto: self, and phage: eat), destroying or recycling its damaged cell (proteins, mitochondria, etc) so that a new and healthy version can be built. However, the field is new and much of the autophagy remains a mystery. Compared with other cell death processes, autophagy induced by radiation or anticancer drugs is less studied.
The current project uses the human mammalian cell lines INT407, HCT116, and HepG 2 as model systems to study autophagy induced by 12C radiation in combination with different types of inhibitors that affect the autophagy mechanism. This study would provide information about radiation-induced autophagy in different types of cells and develop cytotoxic drugs to increase radiosensitivity.
Differential cellular response to 12C beams in normal versus transformed cells with special reference to mitochondria [IUAC]
DNA damage has long been considered as the primary target of radiation causing detrimental effects. Emerging experimental evidence from recent years has accelerated research in understanding mitochondria as an important target for ionizing radiation and its significance in cellular radiation response. Mitochondria are highly dynamic organelles that are continuously undergoing fusion and fission and their function is directly linked to their polarization state. Mitochondria coordinate with other cell organelles and play crucial roles in a wide variety of cellular functions. It will be crucial to understand how alteration in mitochondrial structure, number, and distribution affects cellular function, especially in cells exposed to high LET radiation. It has been shown that proton beam irradiation increases mitochondrial biogenesis and it has been proposed that targeting mitochondrial biogenesis may be a novel inhibitory mechanism for metastatic cancer metabolism. It has also been proposed that modulating mitochondrial dynamics fusion-fission can be a novel approach to enhance proton therapy efficacy in certain kinds of cancers. However, there is not much evidence about the same. The current study intended to understand the effects of carbon beam on mitochondrial biogenesis and mitochondrial fission-fusion homeostasis and also to identify the similarities or/and contrast in the above-mentioned processes in normal versus transformed cells and its modification after 12C beam exposure.
Reporter cells, including HEK 293 cells expressing Su9-EGFP, Mfn2-YFP, RelA- EGFP, and NFkB LacZ are being used to understand changes in mitochondrial function, structure, and NFkB expression after exposure to C beam. Parental HEK 293 (Human embryonic kidney) cells and A498 cells (Kidney Carcinoma) are also being used to study the correlation with the other molecular events happening inside the mitochondria of the cells like changes in overall mitochondrial fission-fusion homeostasis, mitochondrial content, mitochondrial morphology, membrane potential, and oxidative stress after radiation exposure.
Studies on TLR agonist (mannan) Mediated Modification in Biological Radiation Response(s) in vitro after carbon beam exposure [INMAS, DRDO]
Ionizing radiation is known to cause considerable damage to cellular biomolecules, cells, tissues, and organs. To understand the health risk posed by Ionizing Radiation in accidental scenarios, a patient undergoing radiotherapy, and space exploration projects, the research needs to be directed in understanding the molecular mechanism of damage, so that preventive or therapeutic measures can be adopted. The development of safe and effective radiation countermeasure approaches; including radiation protectors, mitigators, and therapeutics are crucial for the management of prospective scenarios (natural, accidental, and/or terrorism) comprising high dose radiation exposure. In recent years, numerous radiation countermeasure agents have been reported, some of which are efficient but relatively toxic. It has been shown that MOS [mannan oligosaccharide] is a potential modulator of biological effects of gamma radiation both at in vitro and in vivo levels. It has been shown that MOS pretreatment in immortalized normal cells reduces the biological effects of γ-radiation and enhances cell survival. MOS, as a radiation countermeasure agent, may also be beneficial in case of planned radiotherapy events in cancer patients, and may also provide health benefits for space crew by minimizing damage caused by cosmic radiation.
The present study aims to examine the effects of MOS in modification in cellular response when exposed to a 12C beam. TLR expressing cells, NKE (Normal Kidney epithelial cells) and A498 cells (Kidney Carcinoma) are being used in the study for evaluation of the predictive radio-protective efficacy of mannan along with the possible mechanism of radiation protection after 12 C beam exposure. The study also aims to understand the mechanistic aspects of radiation response in both radiation protection and mitigation aspects.
Experimental Facilities
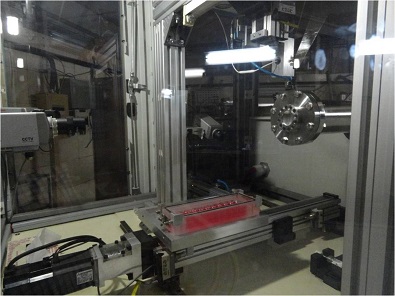
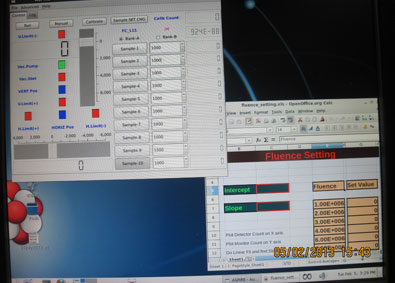
The research facility at IUAC provides a dedicated Radiation Biology Beam line equipped with the irradiation system called ASPIRE [Automatic Sample Positioning for Irradiation in Radiation Biology Experiments].
This is a computer controlled system that enables one to irradiate cells placed on 35 mm petri dishes kept in medium in a sterile condition one after another at quick succession with predetermined particle dose.
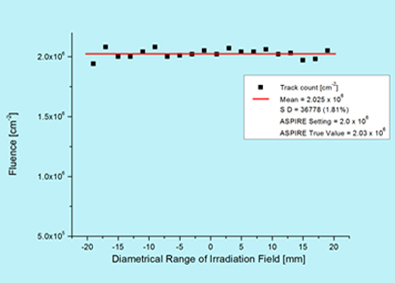
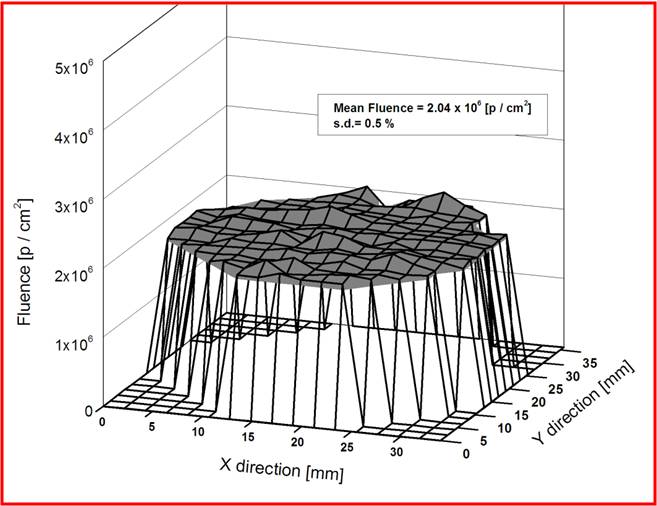
The uniformity as well as fluence verification has been done using irradiating SSNTD [CN-85] and subsequent etching. The tracks were counted using microscope over accurately measured areas across the entire irradiation field of 40 mm diameter. The uniformity over a field of 40 mm diameter is having 2 % standard deviation. The mean fluence is within 1 % of the electronically measured value at the centre of the field.
Photo Gallery
Radiation Biology Beam Line
Important information
| Beam | Beam energy from accelerator [MeV] | Energy on the Cell Surface [MeV] | Corresponding Entrance LET in water [KeV/µm] |
|---|---|---|---|
| 7 Li | 50 | 45 | 60 |
| 11 B | 65 | 48.7 | 218 |
| 12 C | 85 | 62 | 290 |
| 16 O | 100 | 56 | 614 |
All the above values give a tentative picture in order to plan an experiment. The parameters may be adjusted and evaluated during the beam time. The available beam energy also depends on the machine condition during the run.
Particle Flux : 1-2 x 105 particles/cm2 /sec
All the irradiation jobs are done at the atmospheric pressure. The samples have to be prepared in form of a monolayer. The attaching types of mammalian cells are grown on a 35 mm NUNC Petri-dish. The dishes are irradiated in a horizontal position. Non attaching cells can also be irradiated in the horizontal position using a special method. Irradiation with a pre-determined fluence and subsequent repetitions can be done using a the irradiation system called ASPIRE.

Group Members and Contact Details









_contrast_thumb.jpg)
![Coulter Cell Counter [Beckman Coulter],Countess image based Cell Coulter Cell Counter [Beckman Coulter],Countess image based Cell](http://iuac.res.in/themes/iuac/images/cellcount_thumb.jpg)
![CO2 Incubators, [NuAire] CO2 Incubators, [NuAire]](http://iuac.res.in/themes/iuac/images/incub_thumb.jpg)
![Bio safety Cabinet (Class II) [Esco] Bio safety Cabinet (Class II) [Esco]](http://iuac.res.in/themes/iuac/images/biosafe1_thumb.jpg)
![Bio safety Cabinet (Class II) [Heraeus] Bio safety Cabinet (Class II) [Heraeus]](http://iuac.res.in/themes/iuac/images/biosafe2_thumb.jpg)
![Gel Electrophoresis [Stratagene, Tarson], AFIGE [BIORAD], Semi Dry Blotter [BIORAD], PCR [Perkin Elmer] Gel Electrophoresis [Stratagene, Tarson], AFIGE [BIORAD], Semi Dry Blotter [BIORAD], PCR [Perkin Elmer]](http://iuac.res.in/themes/iuac/images/gel_thumb.jpg)
![UV Vis Spectrophotometer [Hitachi] UV Vis Spectrophotometer [Hitachi]](http://iuac.res.in/themes/iuac/images/UV_vis_thumb.jpg)
![PCR [Applied Biosystems] PCR [Applied Biosystems]](http://iuac.res.in/themes/iuac/images/pcr_thumb.jpg)
![96 Well Multi Mode Plate Reader [Perkin Elmer, Victor X5] 96 Well Multi Mode Plate Reader [Perkin Elmer, Victor X5]](http://iuac.res.in/themes/iuac/images/multimode_thumb.jpg)
![Centrifuge [Eppendorf] Centrifuge [Eppendorf]](http://iuac.res.in/themes/iuac/images/centri_thumb.jpg)
![Fluorescence Microscope [Zeiss] Fluorescence Microscope [Zeiss]](http://iuac.res.in/themes/iuac/images/zeiss_thumb.jpg)